You can read 27+ pages arrange the ions by their expected hydration energy explanation in Google Sheet format. Rank These Ions By Their Expected Hydration Energy Al3 Mg2 Na This problem has been solved. The extent of hydration. This energy is called the hydration energy of the cation. Read also arrange and arrange the ions by their expected hydration energy Cu2 has higher hydration energy with respect to Cu1.
10If you were to take gaseous cations and put them into water they would form hydrated ions and release large amounts of energy. The energy basically is released because the bi-polar water molecule stabilizes the ion by ion dipole interactiona form of coulumbic interaction.
The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Arrange the ions by their expected hydration energy Highest hydration energy Lowest hydration energy Answer Bank K Ca.
| Topic: 15The hydration energy of the ions their coordination number and the structure of the water cluster coordinated with the ions were determined. The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Arrange The Ions By Their Expected Hydration Energy |
| Content: Synopsis |
| File Format: DOC |
| File size: 1.6mb |
| Number of Pages: 11+ pages |
| Publication Date: June 2017 |
| Open The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate |
 |
Highest hydration energy Lowest hydration energy K Ga3 Ca2 By signing up.

Rank these ions according to ionic radiusCa2 P3- S2- CI- K. NaOH for example is very soluble in water 420 gL but MgOH 2 dissolves in water only to the extent of 0009 gL and AlOH 3 is essentially insoluble in water. The hydration enthalpies ie energies of hydration of metal ions decreases with increase in ionic radii. The Mg 2 and Sr 2 ions have higher hydration energy. Highest hydration energy Lowest hydration energy Na Mg2 Al3. Rank these ions by their expected hydration energy.

Solution Rank These Ions Their Expect Chemistry Br - Sr2 Rb Se2- As3- Q.
| Topic: Rank these ions by their expected hydration energy from lowest to highest. Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Explanation |
| File Format: PDF |
| File size: 800kb |
| Number of Pages: 17+ pages |
| Publication Date: January 2018 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |
Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes The approach used to calculate ions properties was validated by comparing hydration properties provided by experimental and simulation works.
| Topic: Although this is an experiment that is impossible to perform the hydration energies have been determined indirectly you will see how this is done later in the term. Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes Arrange The Ions By Their Expected Hydration Energy |
| Content: Answer Sheet |
| File Format: PDF |
| File size: 1.8mb |
| Number of Pages: 4+ pages |
| Publication Date: December 2017 |
| Open Emily S Studygram On Instagram Just Finished My Chemistry Exam And It Wasn T As Hard As I Expected It Now I Have 4 More Study Notes Exams Tips School Notes |
 |

Solution Rank These Ions Their Expect Chemistry Rank these ions according to ionic radiusCa2 P3- S2- CI- K Q.
| Topic: Because of the smaller size of Al3 than M g2 and N a its energy of hydration larger than both these ions. Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Learning Guide |
| File Format: DOC |
| File size: 2.2mb |
| Number of Pages: 4+ pages |
| Publication Date: December 2018 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |

Rank These Ions Their Expected Hydration Energy Chegg The first part is the energy released when the solvent forms a coordination compound with the ions.
| Topic: Rank these ions by their expected hydration energy. Rank These Ions Their Expected Hydration Energy Chegg Arrange The Ions By Their Expected Hydration Energy |
| Content: Answer Sheet |
| File Format: Google Sheet |
| File size: 3.4mb |
| Number of Pages: 22+ pages |
| Publication Date: August 2020 |
| Open Rank These Ions Their Expected Hydration Energy Chegg |
 |

Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration NaOH for example is very soluble in water 420 gL but MgOH 2 dissolves in water only to the extent of 0009 gL and AlOH 3 is essentially insoluble in water.
| Topic: Rank these ions according to ionic radiusCa2 P3- S2- CI- K. Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration Arrange The Ions By Their Expected Hydration Energy |
| Content: Solution |
| File Format: PDF |
| File size: 1.9mb |
| Number of Pages: 6+ pages |
| Publication Date: July 2019 |
| Open Arrange Na Mg 2 And Al 3 In Increasing Order Of Energy Of Hydration |
 |

The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate
| Topic: The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate Arrange The Ions By Their Expected Hydration Energy |
| Content: Summary |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 13+ pages |
| Publication Date: April 2019 |
| Open The Enthalpy Of Hydration Of The Fe 2 Ion Is 11 4kcal Mol Higher Than Would Be Expected If There Were No Crystal Field Stabillisation Energey Assuming The Equo Plex To Be High S Estimate |
 |

Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4
| Topic: Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4 Arrange The Ions By Their Expected Hydration Energy |
| Content: Synopsis |
| File Format: DOC |
| File size: 1.6mb |
| Number of Pages: 13+ pages |
| Publication Date: January 2018 |
| Open Petitive Sorption Of Monovalent And Divalent Ions Highly Charged Globular Macromolecules The Journal Of Chemical Physics Vol 153 No 4 |
 |
Solution Rank These Ions Their Expect Chemistry
| Topic: Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Answer Sheet |
| File Format: PDF |
| File size: 1.6mb |
| Number of Pages: 10+ pages |
| Publication Date: October 2017 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |

Solution Rank These Ions Their Expect Chemistry
| Topic: Solution Rank These Ions Their Expect Chemistry Arrange The Ions By Their Expected Hydration Energy |
| Content: Answer |
| File Format: PDF |
| File size: 5mb |
| Number of Pages: 25+ pages |
| Publication Date: October 2017 |
| Open Solution Rank These Ions Their Expect Chemistry |
 |
Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html
| Topic: Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html Arrange The Ions By Their Expected Hydration Energy |
| Content: Learning Guide |
| File Format: Google Sheet |
| File size: 5mb |
| Number of Pages: 55+ pages |
| Publication Date: January 2021 |
| Open Membranes Free Full Text Perfluorosulfonic Acid Membranes Thermally Treated And Modified Dopants With Proton Acceptor Properties For Asparaginate And Potassium Ions Determination In Pharmaceuticals Html |
 |
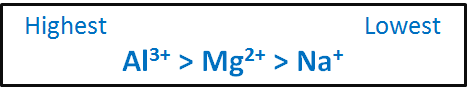
Arrange The Ions Their Expected Hydration Energy Chegg
| Topic: Arrange The Ions Their Expected Hydration Energy Chegg Arrange The Ions By Their Expected Hydration Energy |
| Content: Analysis |
| File Format: PDF |
| File size: 725kb |
| Number of Pages: 17+ pages |
| Publication Date: July 2017 |
| Open Arrange The Ions Their Expected Hydration Energy Chegg |
 |
Its definitely simple to prepare for arrange the ions by their expected hydration energy Emily s studygram on instagram just finished my chemistry exam and it wasn t as hard as i expected it now i have 4 more study notes exams tips school notes the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate arrange na mg 2 and al 3 in increasing order of energy of hydration why does hydration enthalpy decrease on going down a group quora the enthalpy of hydration of the fe 2 ion is 11 4kcal mol higher than would be expected if there were no crystal field stabillisation energey assuming the equo plex to be high s estimate membranes free full text perfluorosulfonic acid membranes thermally treated and modified dopants with proton acceptor properties for asparaginate and potassium ions determination in pharmaceuticals html petitive sorption of monovalent and divalent ions highly charged globular macromolecules the journal of chemical physics vol 153 no 4
